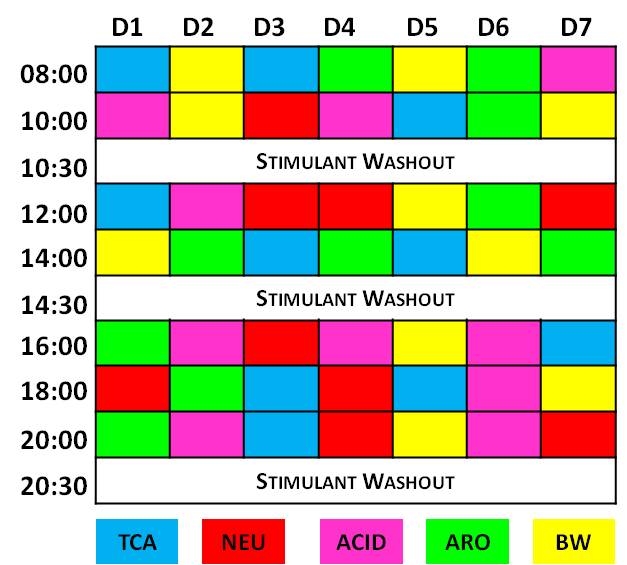
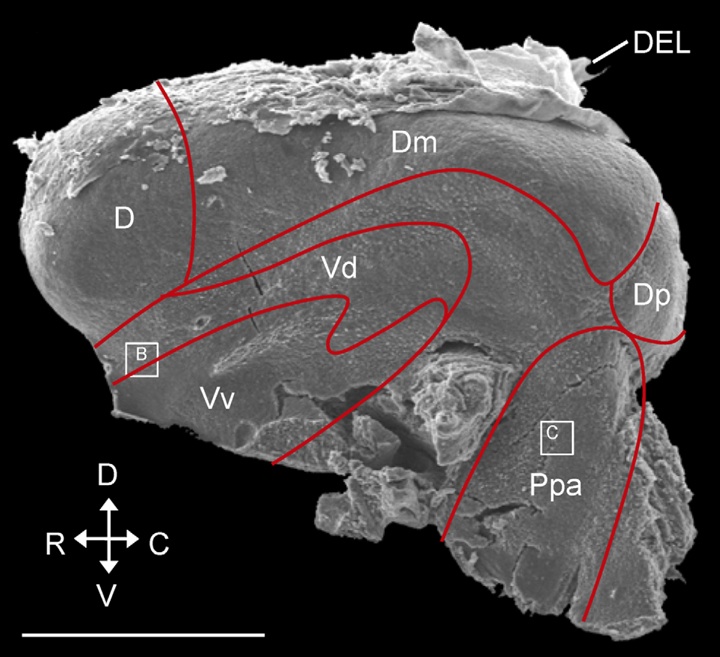
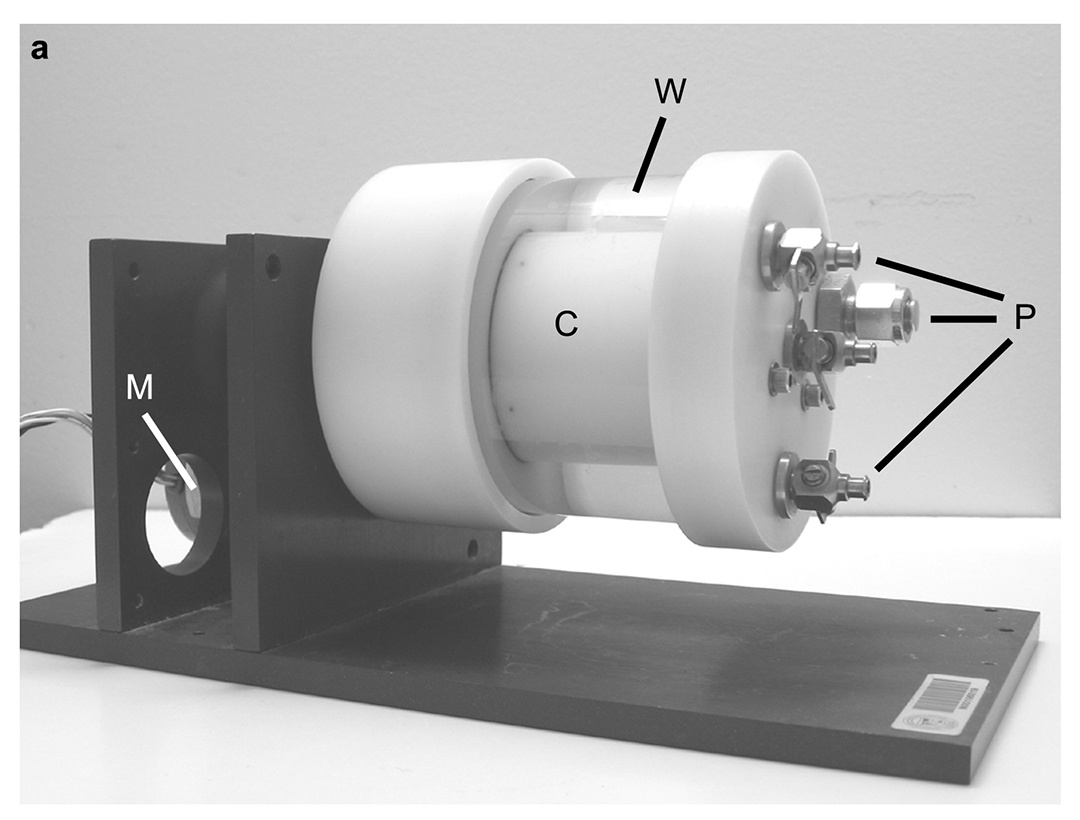
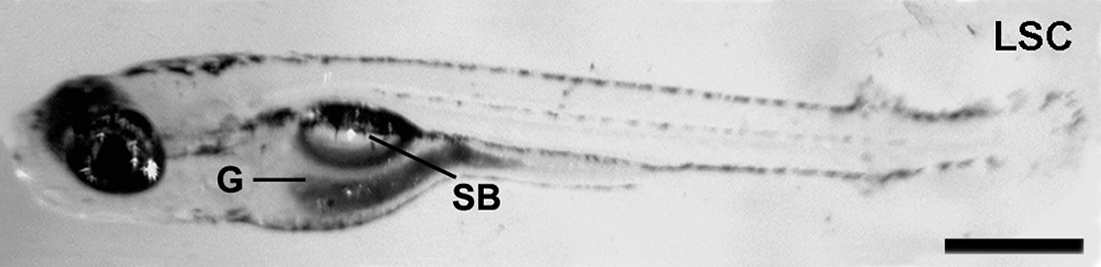
Vertebrate adult neurogenesis can be modulated through changes in proliferation, survival or differentiation, and this appears to be associated with distinct functional requirements for new neurons (Grandel & Brand, 2013).
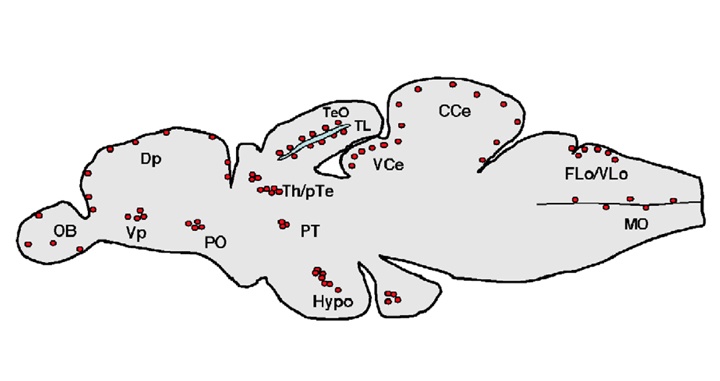
Adult neurogenesis occurs in well-circumscribed regions of the mature vertebrate brain. This process is initiated by the proliferation of stem and/or progenitor cells, which over time generate new neurons and glia.
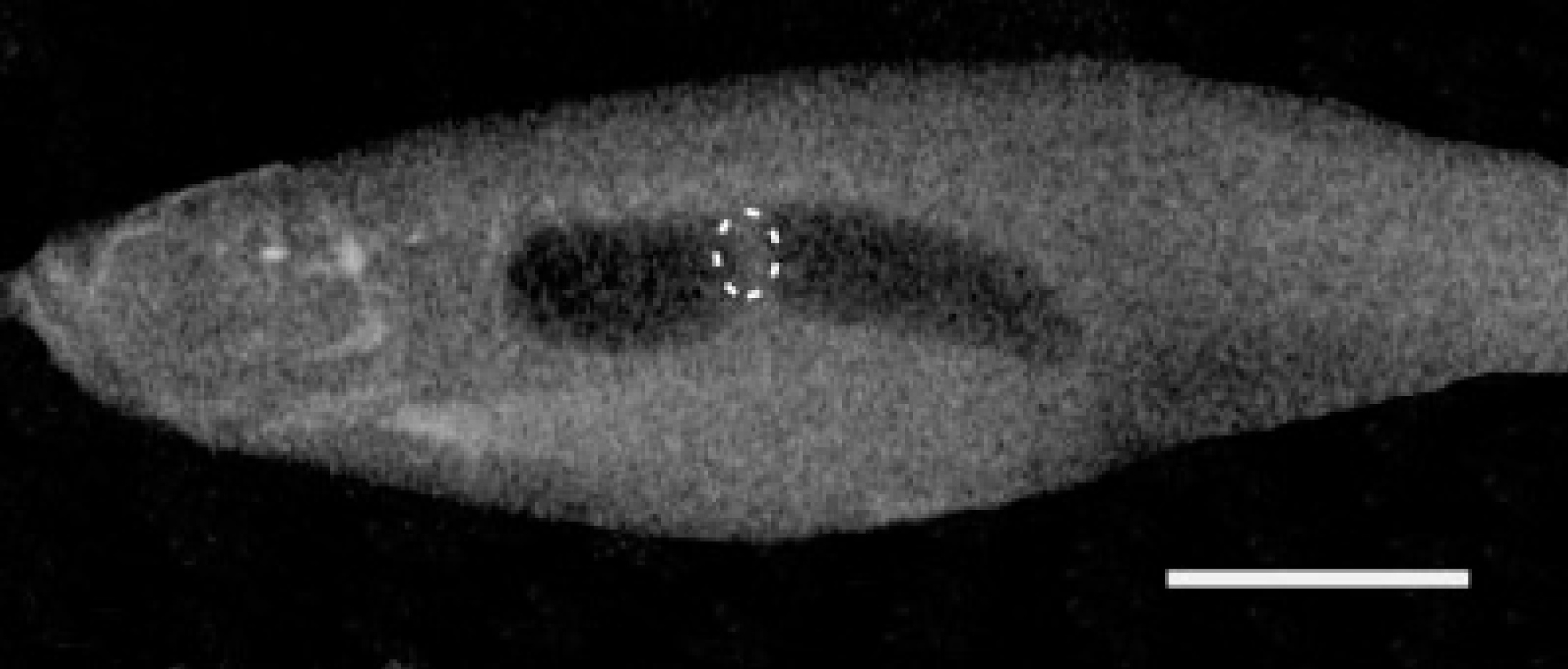
The gas-filled swimbladder of teleost fishes provides hydrodynamic lift which counteracts the high density of other body tissues, and thereby allows the fish to achieve neutral buoyancy with minimal energy expenditure.
Teleost fish contain a variety of tissues, such as muscle, cartilage, and bone, which are denser than the external aqueous environment. Without compensatory mechanisms to provide buoyancy, fish would, therefore, sink.
A significant portion of the body mass of teleost fishes is composed of tissues such as bone and muscle that are denser than water (Alexander, 1993).
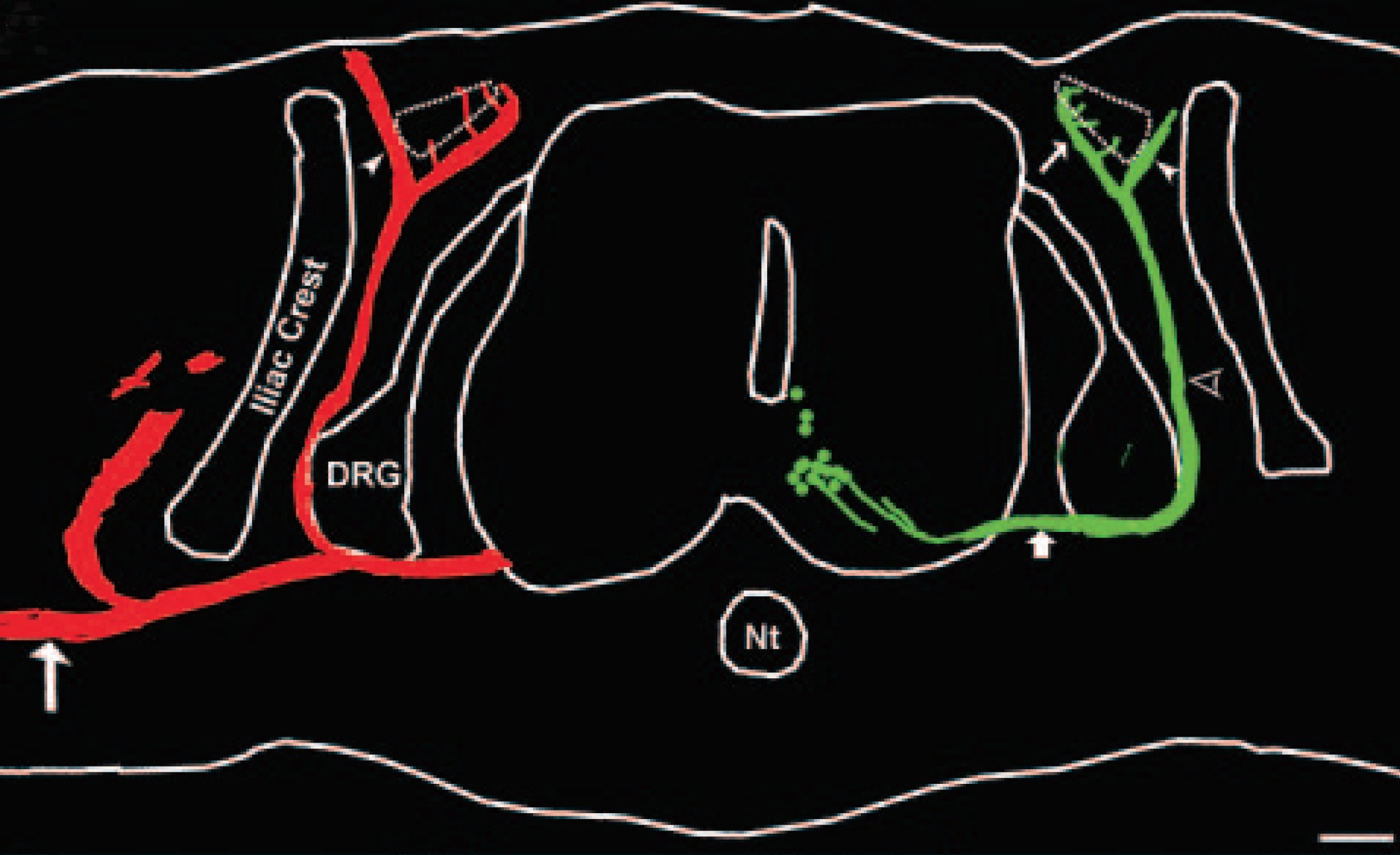
Spinal cord disorders such as amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy result in the dysfunction and eventual death of spinal motoneurons.
Adult neurogenesis appears to be a somewhat extreme and uneconomical form of structural remodeling, compared to the relatively subtle modifications in synaptic morphology that is known to mediate functional plasticity of neural circuitry.
During foraging, animals often feed selectively and choose to pursue or ignore a prey item based on a specific set of pre-determined criteria (Shine and Sun, 2003). Lizards are no exception to this rule, and at present continue to gain popularity for their use as model organisms in ecological studies (Shafir and Roughgarden, 1998).