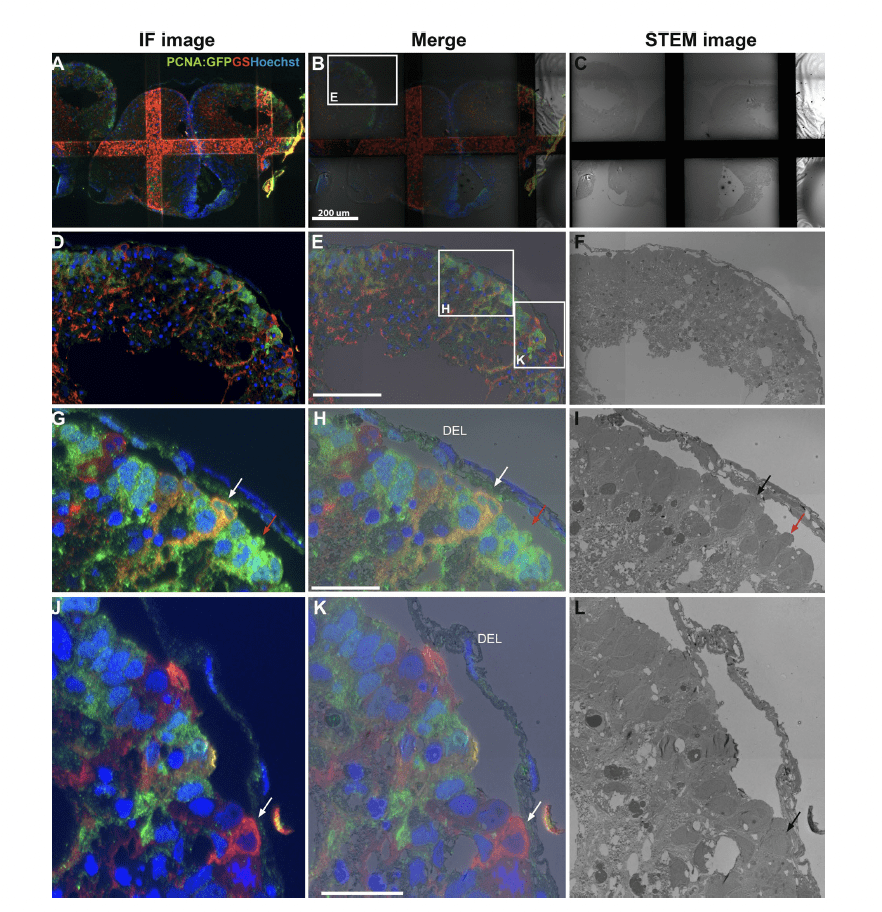
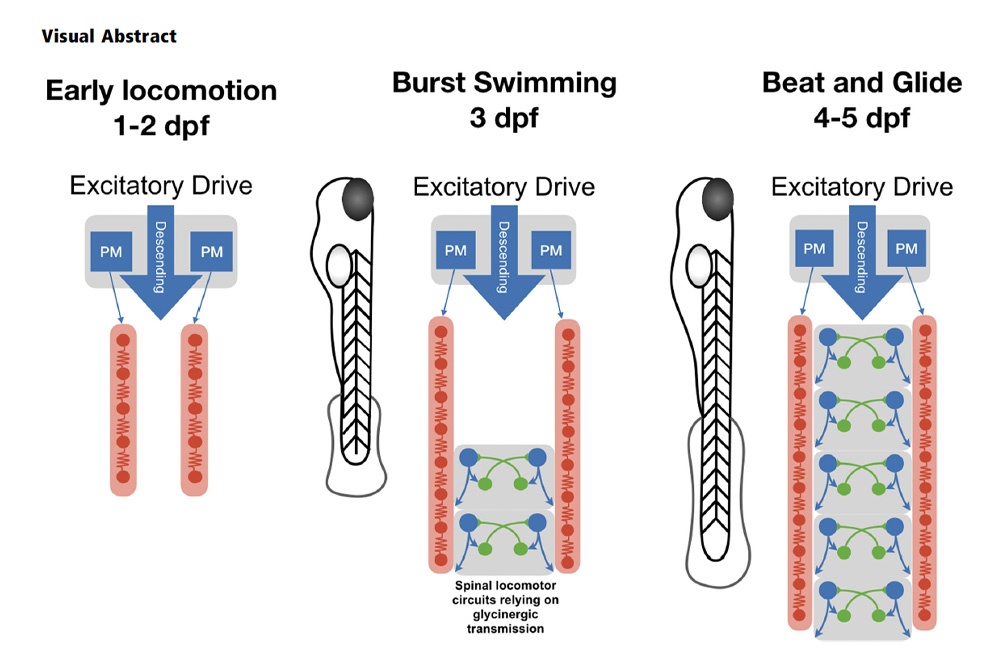
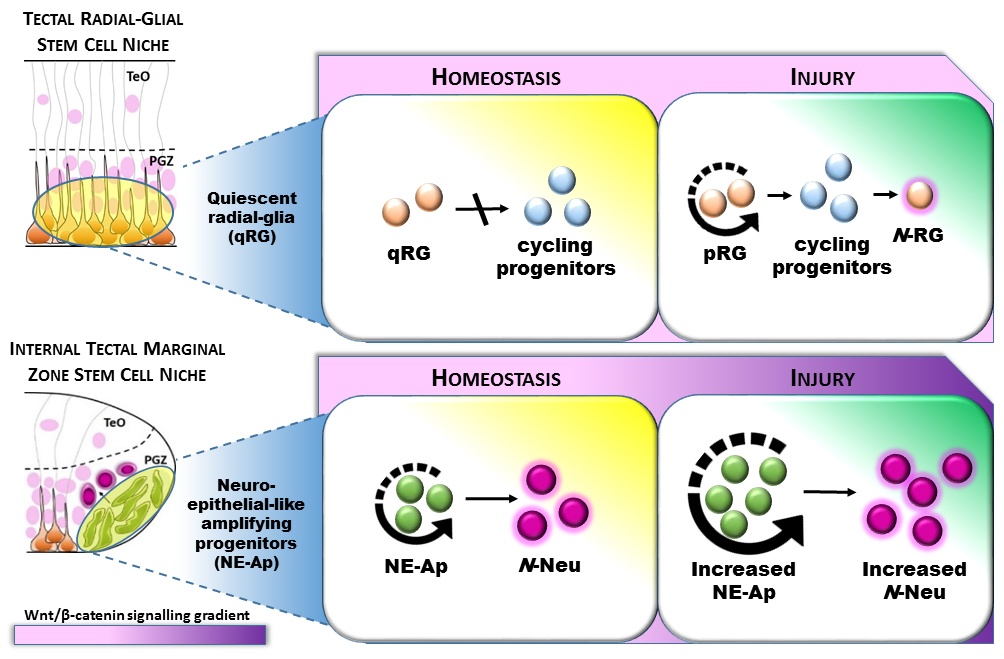
How diverse adult stem and progenitor populations regenerate tissue following damage to the brain is poorly understood. In highly regenerative vertebrates, such as zebrafish, radial-glia (RG) and neuroepithelial-like (NE) stem/progenitor cells contribute to neuronal repair after injury.
How diverse adult stem and progenitor populations regenerate tissue following damage to the brain is poorly understood. In highly regenerative vertebrates, such as zebrafish, radial-glia (RG) and neuroepithelial-like (NE) stem/progenitor cells contribute to neuronal repair after injury.
How diverse adult stem and progenitor populations regenerate tissue following damage to the brain is poorly understood. In highly regenerative vertebrates, such as zebrafish, radial-glia (RG) and neuroepithelial-like (NE) stem/progenitor cells contribute to neuronal repair after injury.
How diverse adult stem and progenitor populations regenerate tissue following damage to the brain is poorly understood. In highly regenerative vertebrates, such as zebrafish, radial-glia (RG) and neuroepithelial-like (NE) stem/progenitor cells contribute to neuronal repair after injury.
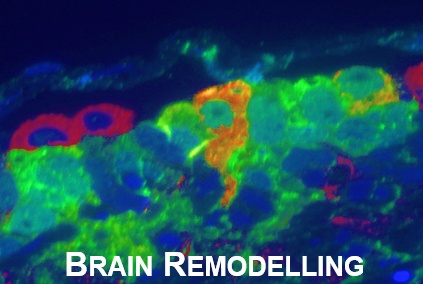
Once thought to be a structurally stable population of glia and neurons arising primarily during early development, the adult central nervous system (CNS) is now known to maintain the capacity to remodel throughout life.
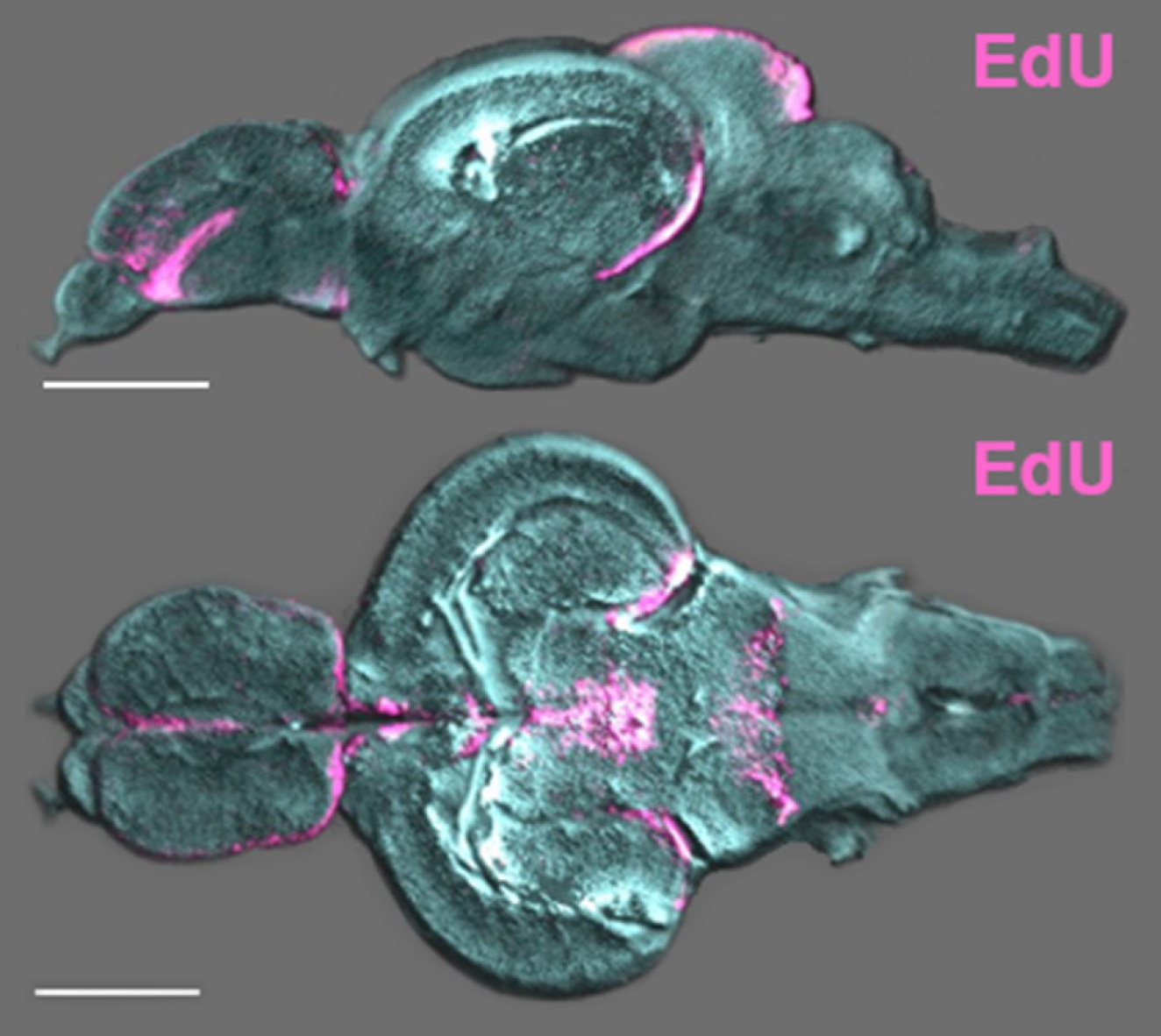
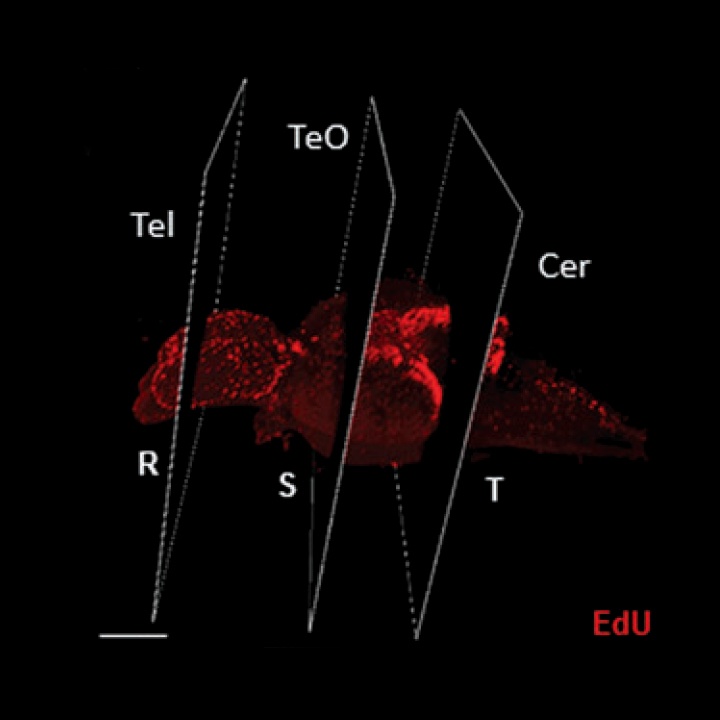
The field of macro-imaging has grown considerably with the appearance of innovative clearing methods and confocal microscopes with lasers capable of penetrating increasing tissue depths.
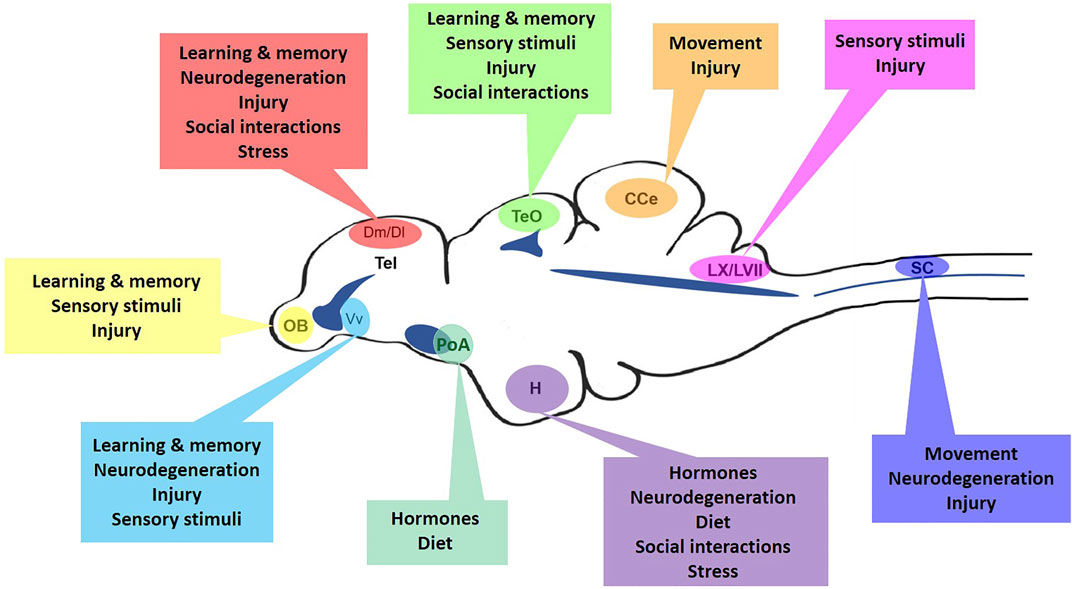
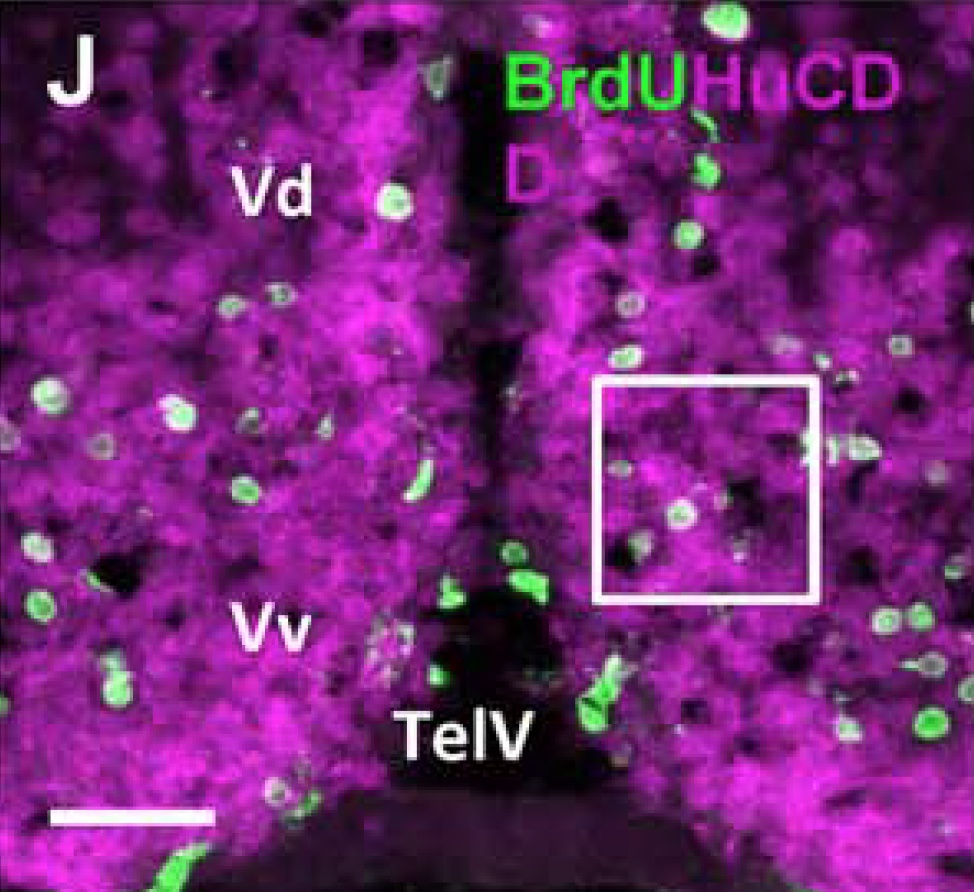
The zebrafish has become a popular model in neuroscience to study changes arising in the mature central nervous system (CNS) as a result of social behaviors, neurogenesis, brain injury and disorders, regeneration, neuroimmune interactions, and neurodegenerative diseases.
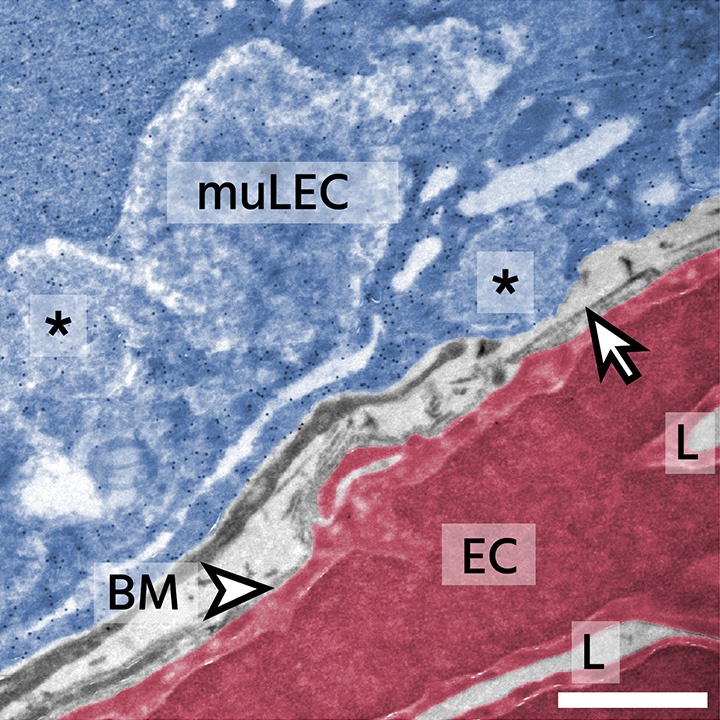
Mural cells of the vertebrate brain maintain vascular integrity and function, play roles in stroke and are involved in maintenance of neural stem cells. However, the origins, diversity and roles of mural cells remain to be fully understood.
Gold nanoparticles (AuNPs) have been widely investigated in drug delivery and imaging. However, for such biomedical applications, the modification of AuNPs is necessary to improve their aqueous dispersion stability and biocompatibility, especially in a salt environment.
The social environment is known to regulate constitutive rates of adult neurogenesis in vertebrates, potentially influencing stem/progenitor proliferation, survival of the post-mitotic population, or the extent of neuronal differentiation (Lindsey and Tropepe, 2006; Kempermann, 2011; Maruska et al., 2012).